Description
Indication:
- Conscience disorder by celphalic injury or brain surgery.
- For promotion of functional recovery of the limbs after hemiplegia resulting from cerebrovascular accidents.
- A combined treatment with anticholinergic drugs for strong tremor symptoms in the Parkinson’s disease or when levodopa becomes ineffective or when occurs the adverse effects of levodopa.
- A combined treatment with antiprotease in treatment of pancreatitis, acute pancreatitis, chronic pancreatitis after surgery.
Contraindication: Hypersensitivity to any component of medicine
Precautions: In cases of intravenous administration, inject very slowly.
Dosage and administration:
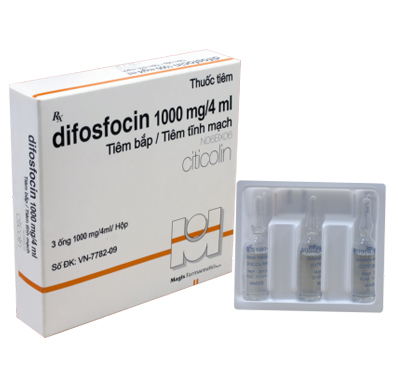
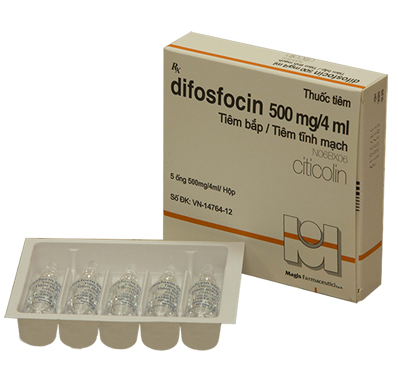
- Consciousness disorders in cerebral diseases and after craniocerebral surgery: Slow intravenous injection, intramuscular or intravenous infusion: 100~500mg citicoline, 1~2 times per day.
- Hemiplegia resulting from cerebrovascular accidents: Intravenous administration of 1,000mg one time a day in 4 weeks consecutively.
- Parkinson’s disease: It shall be given intravenously in an unique dose 500mg per day. After 3~4 weeks, discontinue citicoline and use anticholinergic drugs only.
- Pancreatitis, acute pancreatitis, chronic pancreatitis after surgery: 1000 mg one time a day in 2 weeks consecutively.
Presentation: Ampoule of 4 mL containing 1000 mg Citicoline. Box of 3 ampoules.
Storage condition: Store below 30 degrees C, in dry place, protect from light.
Shelf life: 60 months from manufacturing date
Manufactured by: MITIM S.R.L – ITALY
Holder: MAGIS FARMACEUTICI S.p.A – ITALY
 English
English